An article with the striking title “Africa’s 32 Cents Solution for HIV/AIDS” was recently published in PLoS Neglected Tropical Diseases. It can be seen here:
http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000430
This dramatic title refers to the cost of treatment of schistosomiasis with praziquantal.
Schistosomiasis is an infection caused by parasitic worms, or helminths., of the genus Schistosoma. Most of the 200 million cases of schistosomiasis in the world occur in Africa.
The species, Schistosoma haematobium is estimated to infect about 112 million people in sub Saharan Africa. So its high prevalence puts it in the same class as that of TB, malaria and HIV infection. It is responsible for a huge burden of morbidity particularly in children and young adults. S. haematobium infection predominantly involves the urinary tract. S. mansoni is another species; it affects the intestinal tract.
S. haematobium has a complicated life cycle, some of which takes place in snails. People are infected by organisms released by snails living in fresh water. These organisms can penetrate the skin of any body part that is immersed in snail infested water. The disease it causes is commonly called bilharzia.
I was very conscious of its danger growing up in Zimbabwe, where prominent signs at several small lakes around Bulawayo warned one not to swim in them because of the danger of bilharzia.
There already is a substantial literature that strongly suggests that many endemic infections, not only caused by helminths, but also by bacteria, protozoa, viruses and even common intestinal worms can accelerate the progression of HIV disease and facilitate transmission of the virus . Some of the mechanisms underlying these HIV enhancing effects have been understood for many years. In Europe and N. America the ability of an intercurrent infection to accelerate HIV replication is known from the understanding that viral load testing may show a temporary increase if done during a febrile illness, or even following a vaccination.
The paper cited above, whose lead author is Peter Hotez, concentrates on the local effects of S. haematobium on the female genital tract, where lesions caused by schistosome egg deposition result in mucosal patches, that can bleed during sexual intercourse. The authors state “Presumably, the schistosome egg granulomas produce genital lesions and mucosal barrier breakdown to facilitate HIV viral entry” and go on to compare this to the process by which herpes simplex ulcers increase susceptibility to HIV.
This does seem obvious - there is a mucosal break, so HIV not only has a way in, but will find an accumulation of susceptible CD4 cells at the site of entry. But in this case what appears to be so obvious may not be the important connection between schistosomiasis and susceptibility to HIV infection.
In fact in the case of herpes simplex, this seemingly obvious connection between a genital ulcer and a mode of entry for HIV is probably not correct. The large Partners in Prevention study, recently completed, found that acyclovir, a drug effective in treating herpes does not reduce the risk of HIV transmission. The drug however was associated, as expected, with a reduction in the number of recurrences of herpetic ulcerations, but also appeared to significantly slow the course of HIV disease progression. Since the probability of transmitting HIV is related to viral load then it is quite possible that while acyclovir may not prevent acquisition of HIV it may reduce the infectivity of people harbouring both HIV and a herpes virus.
There are in fact many interconnections between HIV disease and herpesvirus infections, but I will leave a description of these for another post.
As with herpes simplex, it is possible that systemic effects of schistosomiasis, may be much more significant than local effects in enhancing susceptibility to HIV infection. More recently, in direct experiments, acute infection with S. mansoni was shown to increase susceptibility to mucosal infection of Rhesus macaques by SHIV (a monkey adapted virus). Of course, both local and systemic effects may play a role in enhancing HIV transmission.
There are multiple systemic effects associated with many different infections that can accelerate HIV disease progression. Schistosomiasis and some other endemic infections are associated with an impairment of virus specific immune responses. Immune responses may be blunted by several mechanisms. Immune activation will probably also increase susceptibility to HIV and promote its replication. Schistosomiasis and other endemic infectious diseases are also associated with an increased density on the cell surface of the chemokine co-receptors used by HIV which would increase infectability. Pro-inlammatory cytokines such as TNF-alpha and IL 6 that appear during the course of many infectious diseases are potent promoters of HIV replication.
Thus highly prevalent endemic infections can enhance HIV replication by multiple mechanisms. It is likely that this association will also be expressed clinically and has implications for the effective control of HIV in populations where these infections are common.
There have been studies showing that treatment of some of the common endemic infections can lower HIV levels, although it must also be said that not all of the studies agree on this.
Controlling these infections includes inexpensive traditional public health interventions such as supplying clean water and sanitation.
Here is the abstract of an article published in 2006 by W.E.Secor of the CDC, dealing with schistosomiasis, but also applicable to many other infections :
"Interactions between schistosomiasis and infection with HIV-1
In many regions of the world, both schistosomiasis and HIV/AIDS are endemic, resulting in patients harbouring co-infections. Because interaction with host CD4 + T cells is a characteristic of schistosome as well as HIV-1 infections, bi-directional disease effects may be sufficiently different from sequelae caused by either infectious agent alone to warrant alteration of public health approaches in areas of co-endemnicity. Studies published over the past decade provide useful insights into interactions between schistosomiasis and infection with HIV-1, and overall support the hypothesis that special emphasis on treatment of schistosomiasis in populations with elevated prevalence or risk of HIV-1 infection is justified".
Peter Hotez does a great service by continuing to bring attention to a number of devastating neglected tropical diseases. Many have been shown to interact with HIV, but not all by the same mechanisms.
This important article can be seen in the Lancet of May 2nd, 2009, (Lancet 2009 373;1570-1575). I have copied the following table from this article.
The title of the article is:
“Rescuing the bottom billion through control of neglected tropical diseases”
By Peter J Hotez, Alan fenwick, Lorenzo savioli and David Molyneux
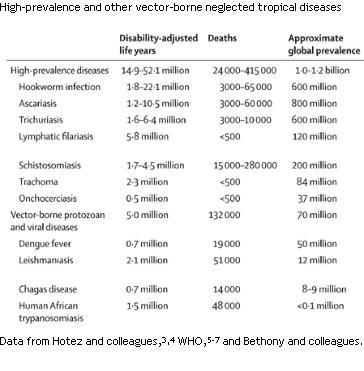
Many of these infections occur in children and young adults and not only can have an impact on life expectancy, but significantly, are the cause of chronic debility particularly in young people.And again, as noted many of these infections also have an activating effect on HIV replication by some mechanisms that have been understood for well over ten years.
Despite a great deal of evidence for the interaction of multiple bacterial, viral, protozoal and helminthic infections with HIV, this association seems to have been relatively neglected as a target in the fight against HIV. Of course we do not need to justify efforts to improve the health of populations by attempting to prevent and treat highly prevalent endemic infectious diseases, but because of disease interactions, funds available to fight HIV could very appropriately be used to combat other infections as well. We can cure and prevent many of these endemic infections, quite often by means that are relatively inexpensive.
To summarize, the health of hundreds of millions of individuals could be improved by efforts to prevent and treat these infections. These infections are also appropriate therapeutic targets in the fight against HIV/AIDS.
HIV disease is not unique in its susceptibility to modification in a host infected with other pathogens. HIV itself can modify the course of other infectious diseases. It may however be unique in that we probably know much more about the mechanisms by which the course of HIV disease can be modified in a host also infected with other disease causing agents. We know much more about interactions between pathogenic micro-organisms through the study of HIV. So this is yet another example of the way HIV research has advanced our general understanding of the pathogenesis of infectious diseases.
Interestingly there are a few examples where the interaction of other infections may temporarily ameliorate HIV disease. Scrub typhus, measles and a form of viral hepatitis, may have a very temporary beneficial effect on HIV disease, but these are exceptional cases. Most co-infections have the opposite effect.
Why have endemic diseases been so neglected in our attempts to control AIDS, particularly in parts of Africa? We have known about some of the interactions for over twenty years; there is no shortage of publications where they have been described. Is it possible that one answer may lie in the lack of effective communication between the different disciplines engaged in HIV research? How do public health experts become informed of advances in microbiology particularly when the field of microbiology itself is changing as a result of new knowledge concerning basic mechanisms? A related issue concerns how funders and policy makers receive the technical information they need to make their decisions.
About two years ago I used a particular article to invite some discussion about these questions.
The title of this article is "Contribution of Immune Activation to the Pathogenesis and transmission of HIV type 1 infection"; the authors are Stephen Lawn, Salvatore Butera and Thomas Folks. (Clinical Microbiology Reviews. Oct 2001 14; 753-777)
This is an account of observations made by microbiologists and work done at a molecular level with enormous implications for the control of AIDS particularly in parts of Africa where co-infections are frequent. The review explains in great technical detail how the replication of HIV can be enormously enhanced by concurrent endemic infections, and how this not only accelerates the progression of HIV disease, but also facilitates its transmission. The authors show in molecular detail how many viral, bacterial, protozoan and helminthic infections can affect HIV replication. Included among these are common intestinal worms and water borne bacterial infections, causing severe diarrhea particularly in infants.
Because of the immunological and molecular detail, I wondered if this review might present some difficulties to those not familiar with the technical terminology and thus the public health implications might not be so evident.. This particular paper is a great illustration of the compartmentalization of information, and the difficulties of interdisciplinary communication.
Here is an illustration from the body of the article: there is much more just like this. A person with no experience of molecular biology or virology would not be likely to spend much time with it.
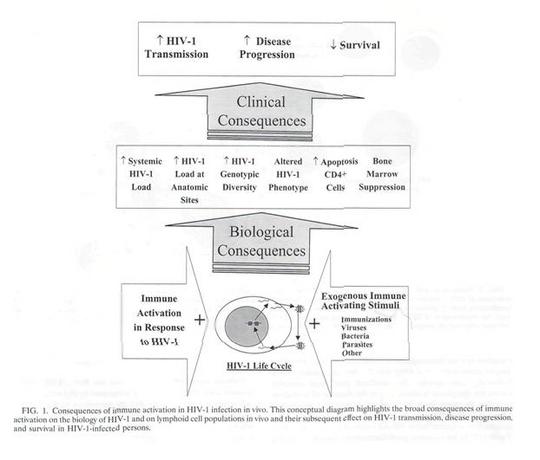
However if one turned a few pages the following diagram might be of some interest.
The part that would be of interest to a public health professional is contained in the large arrow at the bottom right of the illustration. In this rather complex and busy diagram it would be quite easy to be sufficiently distracted so that the bottom right hand corner would be easily missed.
There is a long discussion which is quite technical in nature but at least the authors find space for the following brief comment.
Prevention and Treatment of Coinfections
The widespread use of HAART in the treatment of HIV-
infected persons in westernized countries has resulted in a
phenomenal decrease in the incidence of opportunistic infec-
tions and has greatly increased survival. For these individuals,
the antiretroviral drugs are the major determinant of prognosis
and the potential cofactor effect of opportunistic infections is
now a more minor consideration. However, the vast majority
(>95%) of the world’s HIV-infected people do not currently
have access to antiretroviral drugs. Most of these people live in
developing countries, where the quality and access to health
care is often limited and where there is a high incidence of
endemic infectious diseases such as malaria, TB, and infections
by helminths and waterborne pathogens which may adversely
affect HIV-1 disease progression. Prevention or early treat-
ment of these diseases may therefore represent an important
strategy in addressing the HIV-1 epidemic in developing coun-
tries.
There is quite a long section on possible ways to reduce the immune activation associated with endemic infections, but the last sentence is the only reference that deals with treating and preventing these co-infections.
Clinical Microbiology Reviews where the article appeared is a journal published by the American Society for Microbiology and presumably read mostly by microbiologists. How does the information it contains, as well as an assessment of its relevance find its way to those who advise funders and policy makers and those who inform the public?
In the above quotation, the authors are overoptimistic in their assertion that the HIV enhancing effect of opportunistic infections is now a more minor consideration in Europe and N. America. Valacyclovir, a drug that inhibits the replication of many members of the herpes virus group, but has no direct effect on HIV (unless it is phosphorylated in a cell infected with both viruses, which is unlikely to be of practical significance) was reported to reduce HIV viral loads in the absence of antiretroviral therapy. In the developed world, active herpes virus infections are common in the setting of HIV infection, although most will be asymptomatic. For example, cytomegalovirus, Epstein Barr Virus are not infrequently found to be active in HIV infected individuals. Valacyclovir will have an effect on these viruses, and may well find a place in the treatment of HIV infection in developed countries. Fortunately this drug is already prescribed quite frequently, but often only to those with evidence of herpesvirus type 2 infections, in order to reduce herpesvirus shedding.
Thus endemic infections in Africa have everything to do with HIV/AIDS. There are numerous preventative and therapeutic measures available to control many of these infections. Even something as simple as deworming may be useful. Ascaris lumbricoides, the common intestinal round worm also is associated with immune activation and is easily got rid of. There is a report that doing this with a drug called albendazole actually raised CD4 counts. (Walson JL et al. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. AIDS 22:1601-1609, 2008).
The person who has been studying immune activation and the association of parasitic infestations and AIDS for the longest time is Zvi Bentwich. I can’t remember when his first publication on this issue appeared but by the mid 1990s he was publishing on this association in Ethiopian immigrants to Israel. Zvi Bentwich deserves the greatest credit for his early recognition of the importance of this association, and his continuing contributions. He pointed out the relevance of schistosomiasis to AIDS (and TB) at least 10 years ago. (We share a very long standing interest in the role of alpha interferon in the pathogenesis of HIV disease, also something that I will leave for another post).
The connection of so many endemic infections with AIDS in Africa is also a connection of poverty with AIDS. Of course poverty does not cause AIDS, nor ascariasis, schistosomiasis, tuberculosis, nor any infection. But the acquisition of many endemic infections is facilitated in impoverished populations resulting in lives that are ravaged and shortened.
Many of these infections also interact with HIV to compound the devastation they cause. Poverty, multiple endemic infections and HIV are intimately intertwined and in many instances reciprocally affect and reinforce each other.
If our goal is to improve the health of populations, where multiple infections commonly co-exist, we cannot adequately manage any one of them without also considering the other infections that may be present, both in the individual and in the community.







3 Comments
3 Comments